
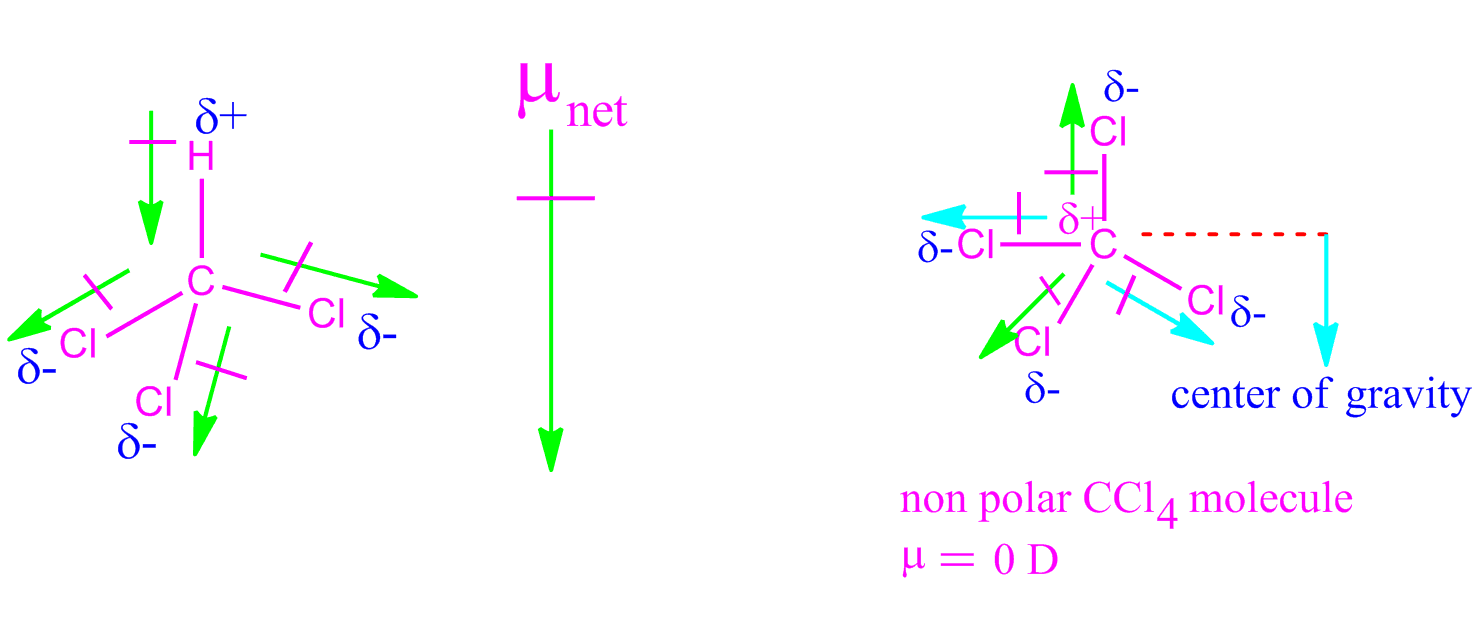
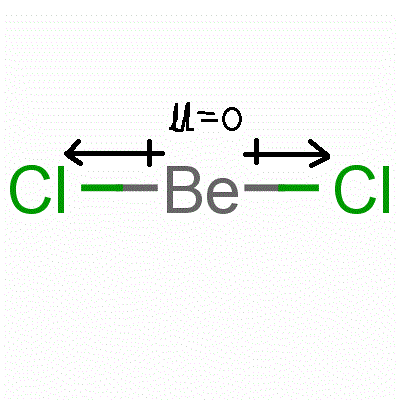
Assign an AX mE n designation then identify the LP–LP, LP–BP, or BP–BP interactions and predict deviations from ideal bond angles.Determine the electron group arrangement around the central atom that minimizes repulsions.Draw the Lewis electron structure of the molecule or polyatomic ion.Using this information, we can describe the molecular geometry, the arrangement of the bonded atoms in a molecule or polyatomic ion. From the BP and LP interactions we can predict both the relative positions of the atoms and the angles between the bonds, called the bond angles.
Each group around the central atom is designated as a bonding pair (BP) or lone (nonbonding) pair (LP). In the VSEPR model, the molecule or polyatomic ion is given an AX mE n designation, where A is the central atom, X is a bonded atom, E is a nonbonding valence electron group (usually a lone pair of electrons), and m and n are integers. That is, the one that minimizes repulsions. Groups are placed around the central atom in a way that produces a molecular structure with the lowest energy. Groups are positioned around the central atom in a way that produces the molecular structure with the lowest energy, as illustrated in Figure 9.1 and Figure 9.2.įigure 9.2Geometries for Species with Two to Six Electron Groups. Because electrons repel each other electrostatically, the most stable arrangement of electron groups (i.e., the one with the lowest energy) is the one that minimizes repulsions. According to this model, valence electrons in the Lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a lone pair of electrons, or even a single unpaired electron, which in the VSEPR model is counted as a lone pair. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence electrons present. Instead, it is a counting procedure that accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair approach. The VSEPR model is not a theory it does not attempt to explain observations. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom.


 0 kommentar(er)
0 kommentar(er)
